Which Of The Following Is A Weak Base?
- Ca(OH)2
- Ba(OH)2
- KOH
- NaOH
- NH3
Answer: NH3
NH3 is a weak base
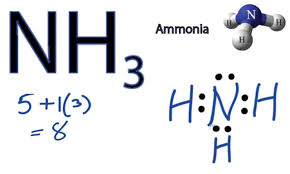
Hopefully, students are familiar with the answer NH3. The answer is quite simple but making choice is not simple at all. You will get the idea that how you will respond according to the NH3 and discussion resolved around it. The NH3 is the weak base because it cannot accept the ions of hydrogen (H plus) in aqueous solution or it is not fully ionized. The weak base can easily donate of valence electrons. NH3 has less or no dramatic effect on Ph. pOH is alternative of PH in the weak base. The same situation with NH3. Kb is the dissociation constant as per the relative strength. NH3 has lowest value of Kb values because it is weak base. Hop you are clear about Which Of The Following Is A Weak Base.

